FDA Approval Marks Historic First in Thalassemia Treatment
In a landmark development for global blood disorder care, the US Food and Drug Administration (FDA) has approved the world’s first oral pill for treating anaemia in adults with thalassemia. The decision has been hailed by health experts as a potential game changer that could significantly reduce lifelong dependence on blood transfusions for patients affected by this inherited condition.
First-of-Its-Kind Oral Therapy
The FDA has approved Mitapivat , to be marketed under the brand name Aqvesme, for adults with both alpha- and beta-thalassemia. Notably, it is the first drug authorised for use in both transfusion-dependent and non-transfusion-dependent patients. Until now, treatment options have largely revolved around repeated blood transfusions combined with iron chelation therapy, often beginning in early childhood.
How Mitapivat Works
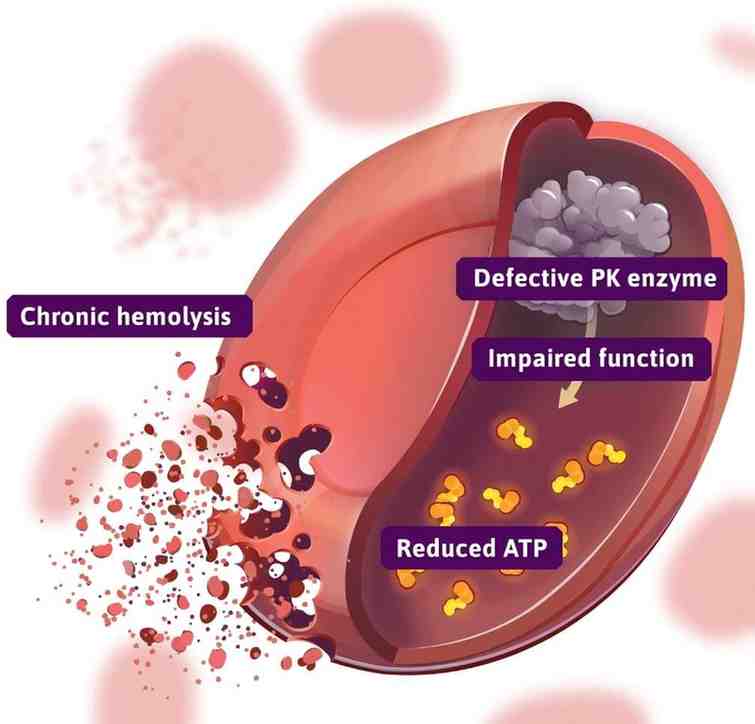
Mitapivat is a first-in-class pyruvate kinase activator that targets the underlying metabolic dysfunction within red blood cells. In thalassemia, red blood cells are fragile and break down prematurely, causing chronic anaemia. By improving energy production inside these cells, mitapivat enhances red blood cell survival, increases haemoglobin levels, and reduces the need for frequent transfusions.
Expert Views on Clinical Impact
Describing the approval as a path-breaking advance, Dr Satyam Arora of the Postgraduate Institute of Child Health, Noida, said the drug offers the possibility of managing thalassemia through a single oral pill. Haematologist Dr Rahul Bhargava noted that this is the first therapy to address the disease at the cellular level rather than merely managing its complications, potentially transforming patient quality of life.
Significance for India’s Disease Burden
India carries one of the highest thalassemia burdens globally, accounting for nearly one-eighth of affected patients worldwide. Experts believe that an effective oral therapy could ease blood shortages, reduce transfusion-related risks, and improve long-term outcomes. Patient groups have expressed hope for early access in India, viewing the approval as a major step towards sustainable and patient-centred care.
Important Facts for Exams
-
Mitapivat is the first oral drug approved for anaemia in adult thalassemia patients.
-
It acts as a pyruvate kinase activator to improve red blood cell survival.
-
The drug is approved for both transfusion-dependent and non-dependent thalassemia.
-
Approval has been granted by the US FDA.
Month: Current Affairs - January 05, 2026
Category: Health | Biotechnology